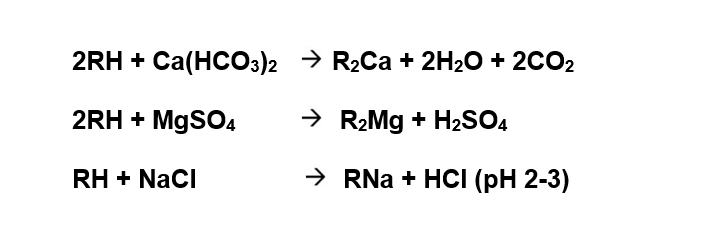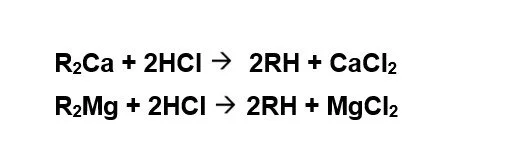Water Demineralization by Ion Exchange
Softening alone is insufficient for most high-pressure boiler feed waters and for many process streams, especially those used in the manufacture of electronics equipment. In addition to the removal of hardness, these processes require removal of all dissolved solids, such as sodium, silica, alkalinity, and the mineral anions (Cl¯, SO4²¯, NO3¯). This is where Demineralization of water is required.
What is Demineralization?
An Ion Exchange Demineralization Plant (IX Demin Plant) is a critical component within industrial and commercial operations, playing a vital role in the removal of impurities and minerals from water. Employing the process of ion exchange, this system facilitates the exchange of ions, culminating in the production of high-quality deionized or demineralized water. The availability of such purified water is indispensable for ensuring the integrity and dependability of end products across a wide spectrum of applications.
Short: Demin Water
Processes involved in Demineralization by Ion Exchange Resin:
Cation exchange: The cation exchange resin bed unit contains strong acid cation exchanger in hydrogen form which converts all the salts present in feed water into acids. Alkaline salts are converted into carbonic acids (H2CO3) while the neutral salts are converted into mineral acids (HCl, H2SO4, HNO3).
Service Cycle Reactions:
Notes:
Outlet water is acidic (pH ~ 2 - 3)
Sodium/hardness leakage is determined
Anion exchange: Anion exchange resin bed neutralizes the mineral acids present in the effluent from the cation unit. This unit contains a strong base anion exchange resin in hydroxyl form. It also removes the residual carbon di-oxide from the degassing unit and weakly ionized silica.
Service Cycle Reactions:
Thus, water obtained at the anion outlet is devoid of any cations and anions. Mostly, a Mixed Bed unit is also employed after both of the units which further purifies water and in some cases acts as a polisher unit.
Regeneration of Resins:
Cation Exchange: On exhaustion, the ion exchange resin is regenerated either by HCl or by H2SO4 Regeneration is carried out either in co-flow or counter-current-flow regeneration mode. Counter-current-flow regeneration mode is preferred since sodium slip from the unit can be reduced to less than 1 mg/l.
Regeneration Reactions:
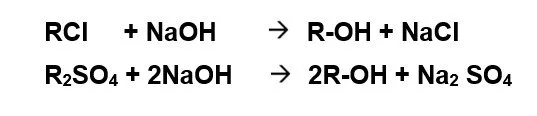
Anion Exchange: On exhaustion, the unit is regenerated by NaOH. Regeneration is carried out either in co-flow or counter-current-flow regeneration mode. Counter-current-flow regeneration mode is preferred since silica slip from the unit can be reduced to less than 0.2 mg/l as SiO2.
Pros and Cons of Water Demineralization by Ion Exchange Resins:
Advantages
Down time due to mechanical failures are less in resin based system.
Electricity consumption is less.
Near to 100% Recovery of Source water is achieved.
Low maintenance is required.
DM water is used in industries needing mineral-free water, like pharmaceuticals, refineries, and power generation.
Disadvantages
Waste water from regeneration of resins needs to be treated in ETP
Resins are sometime fouled with organics ,iron.
Disposal of Waste resin is some time a problem.
Resin beads have to be replaced regularly which can add to operational cost.
Ion exchange waste is highly concentrated and requires careful disposal.
Explore Doshion's industrial water treatment resins for process transformation. Visit Doshion's Industrial Water Treatment Resins page : https://www.doshionpoly.com/ion-exchange-resin